
A Powerful New Treatment in South Africa
for the Pain of Osteoarthritis
Cingal®- Fast Acting.
Long Lasting
Cingal® is a powerful, first-in-class treatment that combines the benefit of Anika’s proprietary high concentration cross-linked hyaluronic acid (HA) formulation, approved for long-term pain relief of the symptoms of Osteoarthritis, with a well-established FDA-approved corticosteroid to treat inflammation and provide additional short-term pain relief.
The Cingal Advantage
- First and only approved HA plus corticosteroid combination viscosupplement*
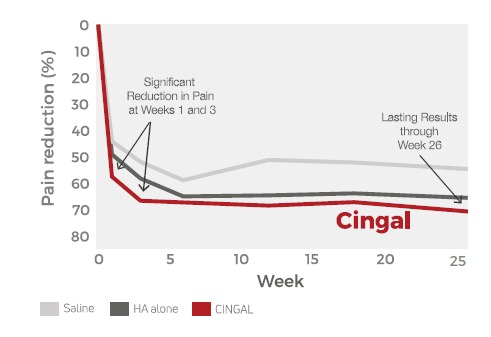
- Provides fast-acting pain relief within days of injection
- Long-lasting pain relief up to six months
- Strong safety profile in both an initial injection and repeat injection
- High delivered dose of cross-linked hyaluronic acid (88 mg) plus 18 mg triamcinolone hexacetonide (TH) in a convenient single injection treatment
- Highly concentrated, non-animal-based HA
- CE Mark approved for use in the knee
WHAT IS VISCOSUPPLEMENTATION?
Viscosupplementation represents an effective, safe, convenient, and non-surgical therapeutic alternative or adjunct to physical therapy, medication or surgery. Viscosupplementation is a procedure where hyaluronic acid (HA) is injected into the synovial fluid surrounding the knee joint for the treatment of the pain associated with osteoarthritis. HA acts as a joint lubricant, providing pain relief and increased mobility.
Intra-articular HA injections have been used for over 25 years1 to treat the symptoms of OA with minimal side effects. Prolonged use of acetaminophen and non-steroidal anti-inflammatories (NSAIDs) can put the patient at risk for significant side effects of liver toxicity (acetaminophen)2 and severe gastrointestinal complications.3 In addition, pharmacological management of OA could result in progressive damage to the joint due to the focus on the reduction of stress-induced pain without additional viscous efficacy.4
HIGHLY VISCOUS SOLUTION
HIGHLY VISCOUS SOLUTION
The primary and immediate benefit of viscosupplementation is the replacement of HA in the synovial fluid. The goal is to improve the viscoelasticity of the synovial fluid, allowing it to function as a viscous lubricant or an elastic material under varying load conditions and mechanical stresses.5 The injection of hyaluronic acid into the joint space results in both the reduction of pain and improved mobility in the treated knee joints.6
CINGAL creates a high viscosity environment within the joint, delivering improved elasticity to the synovial fluid and providing cushioning and joint protection.
HIGH CONCENTRATION OF HA
HIGH CONCENTRATION OF HA
HA is a major component of the extracellular matrix and is naturally found in high concentrations in the synovial fluid surrounding joints. The viscoelasticity of the synovial fluid is determined by both the concentration and the molecular weight of the HA. CINGAL is formulated with a high concentration of HA with a total delivered dose of 88 mg, intended to mimic the high concentration of HA found in healthy synovial fluid.
CINGAL combines a high concentration HA formulation with triamcinolone hexacetonide (TH). Unlike other steroids with shorter duration, TH lasts in the joint knee for 21 days.7
MOLECULAR WEIGHT MATTERS
The high molecular weight of HA gives the synovial fluid its essential viscous and lubricating properties. It is important that the molecular weight of HA is not too low, but not excessively high.8
Optimal high molecular weight HA results in greater pain reduction and longer duration of effect than low molecular weight or excessively high molecular weight. 9 CINGAL has an optimal high molecular weight HA averaging 1.9 million Daltons.8
NATURALLY-SOURCED
CINGAL is a transparent gel made from a natural substance called sodium hyaluronate, also known as hyaluronan or hyaluronic acid (HA), produced by bacterial fermentation. Other viscosupplementation products contain HA derived from rooster combs, which increases patients’ risk of developing allergic reactions to animal proteins
CROSS-LINK TECHNOLOGY
Since naturally occurring hyaluronic acid (HA) is quickly degraded and resynthesized by the body, most HA-based therapeutics require stabilization. A common way to stabilize HA is to bind, or cross-link, HA polymers to other HA polymers, which functions to prevent their rapid, natural degradation.
Anika Therapeutics has developed a proprietary chemical modification process for cross-linking HA chains, creating molecular stability and enabling heat sterilization. This leads to a more durable material that remains in the body for a longer period and has enhanced mechanical properties.10
When cross-linked, the HA polymers increase in viscosity (thickness), and they convert from a liquid to a gel. The viscosity of this gel is determined by several factors, including the degree of cross-linkage between the HA chains. Cross-linked HA chains are still degraded by the body, but the degradation process takes several months, as opposed to the several hour processes for HA polymers that are not cross-linked.
CINGAL is made up of hyaluronic acid that is lightly cross-linked, which extends the residence time of the HA molecules in the knee joint space, providing pain relief that lasts longer.
CINGAL® combines a fast acting steroid with Anika’s proprietary cross-linked hyaluronic acid formulation in one single injection treatment to provide rapid pain relief that lasts.
More about Cingal
Interested in booking a consultation or demonstration? Get in touch with us today!
1. Ault A. From the Head of a Rooster To a Smiling Face Near You. New York Times, 2003 Dec.
2. Stirnimann, G., K. Kessebohm, and B. Lauterburg, Liver injury caused by drugs: an update. Swiss Med Wkly, 2010. 140: p. w13080.
3. Gabriel, S.E., L. Jaakkimainen, and C. Bombardier, Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Annals of internal medicine, 1991. 115(10): p. 787-96.
4. Frizziero L, Govoni E, Bacchini P. Intra-articular hyaluronan in the treatment of osteoarthritis of the knee: clinical and morphological study. Clin Exp Rheum 1998; 16: 441–9.
5. Anika data on file
6. Brandt K., Block J., Michalski J., Moreland L., Caldwell J., Lavin P. Efficacy and safety of intra-articular sodium hyaluronate in knee osteoarthritis. Clinical Orthopaedics and Related Research 2001;(385): 130-143.
7. Stephens M., et. al. Musculoskeletal Injections: A Review of the Evidence. Am Fam Physician. 2008 Oct 15;78(8):971-976. Web: http://www.aafp.org/afp/2008/1015/p971.html
8. DePuy Mitek, Inc. Clinical Efficacy and Safety of MONOVISC® High Molecular Weight Hyaluronan. DSUS/MTK/0714/0232 08/14. 2014.
9. Balazs, E.A. (1974). The physical properties of synovial fluid and the special role of hyaluronic acid. In Disorders of the Knee. (Ed. Helfet, A.) T.B. Lippincott
10. Anika Proprietary data on file
